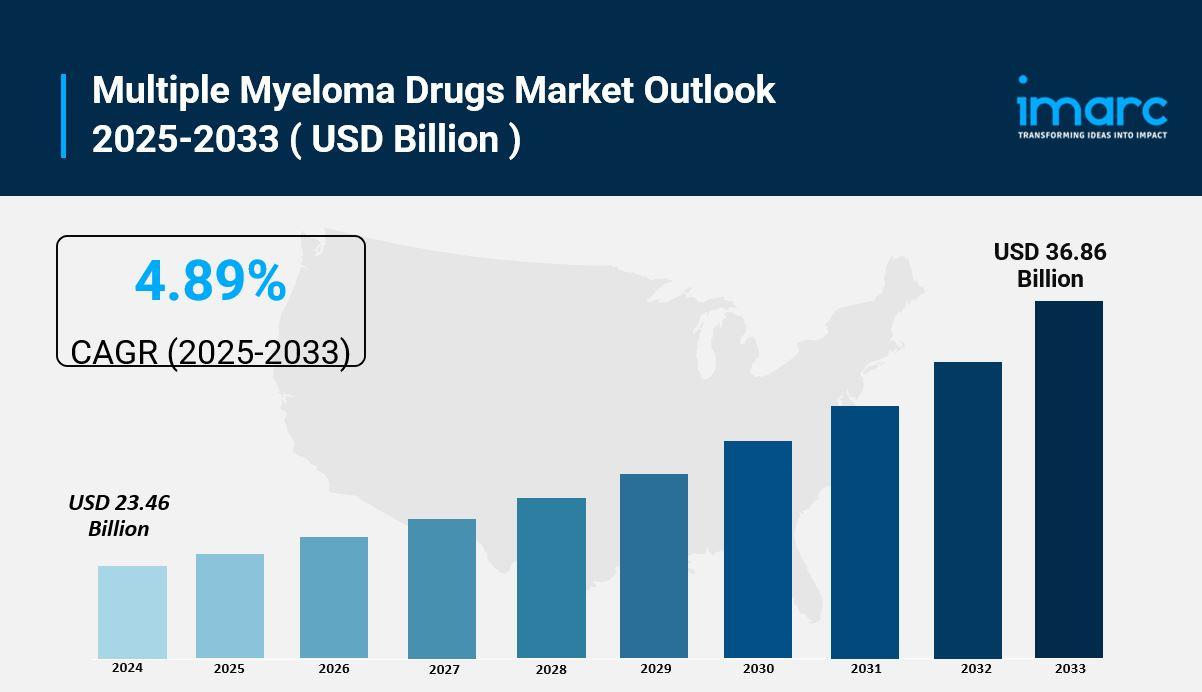
The global Multiple Myeloma Drugs Market size was valued at USD 23.46 Billion in 2024. It is projected to reach USD 36.86 Billion by 2033, growing at a CAGR of 4.89% during 2025-2033. Growth is driven by advances in precision medicine, biomarker-driven treatments, novel drug discovery including bispecific antibodies, and improved clinical trial designs enabling faster market access.
The global multiple myeloma drugs market share is expanding steadily due to the rising prevalence of multiple myeloma, increasing awareness of early diagnosis, and advancements in targeted therapies and immunotherapies. Pharmaceutical companies are investing heavily in research and development to introduce innovative treatment options with improved efficacy and fewer side effects. The growing adoption of combination therapies and personalized medicine further supports market growth. Additionally, supportive government initiatives and rising healthcare expenditure are enhancing patient access to advanced treatments. As novel drug approvals continue, the multiple myeloma drugs market is poised for significant growth in the coming years.
Study Assumption Years
- Base Year: 2024
- Historical Year/Period: 2019-2024
- Forecast Year/Period: 2025-2033
Multiple Myeloma Drugs Market Key Takeaways
- Current Market Size: USD 23.46 Billion in 2024
- CAGR: 4.89%
- Forecast Period: 2025-2033
- North America dominates with over 40.7% market share in 2024 owing to advanced healthcare infrastructure and oncology research investments.
- Rising incidence of hematological cancers and increasing adoption of targeted and biologic therapies are major growth drivers.
- Hospital pharmacies hold around 57.0% of the market share due to specialized drug administration needs.
- Immunomodulatory drugs segment leads with approximately 38.7% market share, driven by effectiveness in modulating immune responses.
- Strategic collaborations and technological advancements in nanomedicine and CAR-T therapies strengthen market growth.
Request Sample: https://www.imarcgroup.com/multiple-myeloma-drugs-market/requestsample
Market Growth Factors
Rising Burden of Hematological Cancers Boosting Drug Demand
The increasing incidence of hematologic malignancies significantly influences the multiple myeloma drug market. In 2022, hematologic cancers accounted for 6.6% of all diagnosed cancer cases and 7.2% of related deaths globally. Because multiple myeloma often originates from genetic abnormalities, its treatment necessitates a multidisciplinary approach including immunomodulatory drugs, chemotherapy, radiation, and stem cell transplants. Early detection improvements have increased patient eligibility for advanced treatments, pressuring healthcare systems worldwide to adopt effective therapeutics, thereby accelerating innovation and investment in myeloma-specific drugs and enhancing market prospects.
Technological Advancements Accelerating Treatment Innovations
Technological progress such as microRNA-based therapies and nanomedicines is transforming treatment by enabling precision targeting of malignant cells while sparing healthy tissues. A notable example is Ardena’s nanomedicine facility in Oss, Netherlands, which secured GMP certification in November 2024 after a €20 million investment. This facility manufactures lipid and polymeric nanoparticles that deliver macromolecular agents to bone marrow, triggering antitumor responses. Such innovations improve outcomes and reduce long-term healthcare costs by minimizing complications. Pharmaceutical companies expanding nanotech capabilities expect these advances to drive market growth significantly.
Increasing Biologic Therapy Adoption and Healthcare Spending
Growing awareness and adoption of biologic therapies, which harness the immune system to eliminate cancer cells, are reshaping treatment preferences for multiple myeloma. These therapies offer targeted mechanisms with fewer systemic side effects, attracting patient interest. Additionally, global healthcare expenditures amounted to USD 9.8 Trillion in 2021 (10.3% of GDP), facilitating wider access to advanced treatments. Coupled with increasing pharmaceutical research budgets, biologics are gaining traction in developed and emerging markets, supporting expanded market growth in multiple myeloma drugs.
Market Segmentation
Analysis by Therapy:
- Targeted Therapy: Offers precision-based approaches by interfering with specific molecules responsible for cancer cell growth, showing enhanced efficacy and fewer side effects.
- Biologic Therapy: Utilizes the immune system to combat cancer, with monoclonal antibodies like daratumumab showing strong clinical success.
- Chemotherapy: Fundamental treatment, especially for newly diagnosed or relapsed patients, maintaining relevance through combination regimens and new formulations.
- Others
Analysis by Drug Type:
- Immunomodulatory Drugs: Largest segment with 38.7% market share in 2024, effective in enhancing immune responses and directly targeting cancer cells; includes lenalidomide and pomalidomide.
- Proteasome Inhibitors
- Histone Deacetylase Inhibitors
- Monoclonal Antibody Drugs
- Steroids
- Others
Analysis by End User:
- Men: Higher prevalence and diagnosis rate increases market demand.
- Women: Growing awareness and access, with expanding screening and research tailored to physiological responses.
Analysis by Distribution Channel:
- Hospital Pharmacies: Lead market with around 57.0% share due to complex treatment regimens needing specialized administration.
- Retail Pharmacies
- Online Pharmacies
- Others
Regional Insights
North America dominates the multiple myeloma drugs market with over 40.7% market share in 2024. The region benefits from advanced healthcare infrastructure, the presence of major pharmaceutical firms, early adoption of innovative therapies, robust regulatory support, and substantial investment in oncology research. These factors collectively reinforce North America’s leadership position and sustained growth in this market segment.
Recent Developments & News
- June 2025: EMA’s CHMP gave a positive opinion for DARZALEX (daratumumab) SC monotherapy for adults with high-risk smouldering multiple myeloma, supported by Phase 3 AQUILA results.
- April 2025: Amneal and Shilpa launched BORUZU, the first ready-to-use subcutaneous or IV bortezomib in the U.S., enhancing pharmacy efficiency.
- March 2025: Bristol Myers Squibb acquired 2seventy bio for USD 286 Million, gaining full ownership of the Abecma cell therapy.
- January 2025: FDA approved daratumumab (Darzalex Faspro) and isatuximab (Sarclisa) combined with VRd for newly diagnosed multiple myeloma patients.
- January 2025: China’s NMPA approved Sarclisa with pomalidomide and dexamethasone for adults with relapsed or refractory multiple myeloma.
- November 2024: Roche agreed to acquire Poseida Therapeutics for up to USD 1.5 Billion, expanding its cell therapy pipeline.
Key Players
- Amgen Inc.
- Bristol Myers Squibb
- Daiichi Sankyo Co., Ltd.
- Sanofi-Aventis Groupe (Genzyme Corporation)
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Novartis International AG
- Pfizer Inc.
- PHARMA MAR, S.A.
- Takeda Pharmaceutical Company Limited.
- Teva Pharmaceutical Industries Ltd.
Customization Note
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Ask An Analyst: https://www.imarcgroup.com/request?type=report&id=2169&flag=C
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA,
Email: sales@imarcgroup.com,
Tel No: (D) +91 120 433 0800,
United States: +1-201971-6302
Join our community to interact with posts!