IMARC Group, a leading market research company, has recently released a report titled "Mycoplasma Testing Market Report by Product (Instruments, Kits and Reagents, Services), Technology (NAAT (Nucleic Acid Amplification Technique), ELISA (Enzyme-linked Immunoassay), DNA Staining, and Others), Application (Cell Line Testing, Virus Testing, End of Production Cells Testing, and Others), End User (Academic Research Institutes, Cell Banks, Contract Research Organizations, Pharmaceutical and Biotechnology Companies, and Others), and Region 2025-2033." The study provides a detailed analysis of the industry, including the global mycoplasma testing market size, share, trends, growth and forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Mycoplasma Testing Market Overview
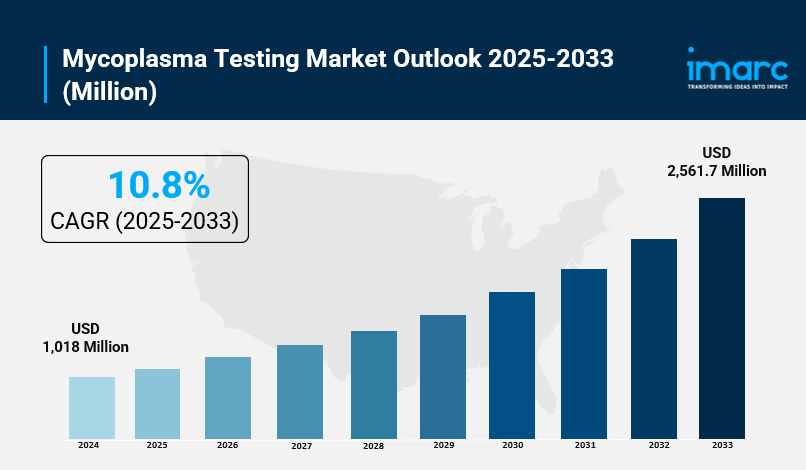
The global mycoplasma testing market size was valued at USD 1,018 Million in 2024. It is projected to reach USD 2,561.7 Million by 2033, growing at a CAGR of 10.8% during the forecast period of 2025-2033. The market growth is driven by increasing prevalence of mycoplasma infections, expansion of the biotechnology industry, stringent testing guidelines, and rising disposable incomes in developing countries.
Study Assumption Years
- Base Year: 2024
- Historical Year/Period: 2019-2024
- Forecast Year/Period: 2025-2033
Mycoplasma Testing Market Key Takeaways
- Current Market Size in 2024: USD 1,018 Million
- CAGR during 2025-2033: 10.8%
- Forecast Period: 2025-2033
- Kits and reagents represent the largest product segment due to demand for ready-to-use components simplifying testing processes.
- NAAT (Nucleic Acid Amplification Technique) technology is the most attractive, offering high sensitivity and rapid results.
- Cell line testing is the dominant application, essential for verifying contamination-free cell lines.
- Pharmaceutical and biotechnology companies account for the largest end-user share in the market.
- North America holds the largest regional market share owing to well-developed biotech infrastructure and active research organizations.
Request Your Free “Mycoplasma Testing Market” Insights Sample PDF: https://www.imarcgroup.com/mycoplasma-testing-market/requestsample
Market Growth Factors
The global rise in the incidence of mycoplasma infections, such as respiratory tract, urogenital tract, and systemic infections, has led to a rise in the demand for mycoplasma infection diagnosis and is driving the market. Drug contamination with mycoplasma and contamination of production processes have encouraged common mycoplasma testing in the healthcare and biotechnology industries.
The stringent regulatory guidelines implemented by several countries to ensure compliance with product approvals and product quality to help promote the adoption of mycoplasma testing procedures. The rapid commercialization of highly sensitive and specific polymerase chain reaction (PCR)-based testing procedures for detecting contamination is expected to increase the industry growth.
The market is increased by rapid growth in the biotech and biopharmaceuticals industries. Manufacturers have to test for mycoplasma in large-scale cell culture to maintain cell health and ensure production consistency. The increased adoption of advanced testing technologies and partnerships with laboratories to meet growing demand further highlight the market's positive growth scenario.
Market Segmentation
Breakup by Product:
- Instruments: Used primarily by research laboratories and biotech companies for in-house molecular testing, driving demand for efficient, high-throughput instruments.
- Kits and Reagents: Largest segment; includes ready-to-use kits with pre-optimized protocols simplifying testing processes for laboratories.
- Services: Growing due to outsourcing trends by large companies to specialized providers ensuring compliance and reliable testing.
Breakup by Technology:
- NAAT (Nucleic Acid Amplification Technique): Includes PCR; high sensitivity and specificity with quick results drives market adoption.
- ELISA (Enzyme-linked Immunoassay): Widely adopted immunoassay, easy to use, cost-effective, and capable of detecting specific mycoplasma antigens across sample types.
- DNA Staining: Simple, cost-effective approach; often used complementary to other methods like NAAT or ELISA.
- Others:
Breakup by Application:
- Cell Line Testing: Dominant application; verifies contamination-free cell lines to ensure integrity in biotech applications.
- Virus Testing: Increasing use for detecting viral contamination in vaccines, therapeutic proteins, and gene therapy, mandated by regulatory bodies.
- End of Production Cells Testing: Used for quality and purity verification before downstream processing, offering market opportunities.
- Others:
Breakup by End User:
- Academic Research Institutes: Conduct extensive studies involving cell cultures, driving market demand.
- Cell Banks: Serve as repositories and suppliers of diverse cell lines crucial for research and manufacturing.
- Contract Research Organizations: Provide high-quality R&D services handling various biological samples, mainly for pharmaceutical and biotech firms.
- Pharmaceutical and Biotechnology Companies: Largest segment; heavily use mycoplasma testing for quality assurance.
- Others:
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America dominates the global mycoplasma testing market, attributed to its advanced biotechnology and life science research infrastructure. The region benefits from numerous pharmaceutical, biotechnology, and research organizations actively involved in drug, vaccine, and biologics development. Regulatory authorities in the USA mandate extensive testing which, combined with ongoing technological advancements and robust R&D, sustains significant market growth.
Purchase the 2026 Report Version: https://www.imarcgroup.com/checkout?id=12997&method=1670
Recent Developments & News
- In July 2018, ATCC launched a PCR-based mycoplasma detection service (ATCC 136-XV) using Whatman FTA sample collection cards combined with the Universal Mycoplasma Detection Kit, enabling detection of over 60 mycoplasma species common in cell culture.
- On December 15, 2021, Asahi Kasei Medical acquired Bionique, a US-based mycoplasma testing services company, contributing to expansion of biosafety testing services.
- In April 2021, Bio-Rad introduced ddPCR Assays for AAV Viral Titer and Vericheck ddPCR Mycoplasma Detection Kit on Droplet Digital PCR platforms to support safe cell and gene therapy development.
Key Players
- ATCC
- Bionique Testing Laboratories Inc. (Asahi Kasei Medical Co. Ltd.)
- Bio-Rad Laboratories Inc.
- Charles River Laboratories International Inc.
- Eurofins Scientific SE
- Invivogen
- Lonza Group
- Merck KGaA
- Roche Holding AG
- Sartorius AG
- Thermo Fisher Scientific Inc.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA,
Email: sales@imarcgroup.com,
Tel No: (D) +91 120 433 0800,
United States: +1-201971-6302
Join our community to interact with posts!